Clinical research is the foundation upon which life-changing treatments are built in the quest for medical breakthroughs. But what fuels this critical work? The answer lies in clinical data management. High-quality data is the unsung hero of medical advancements, enabling researchers to make informed decisions, drive innovation, and save lives. In this article, we’ll delve into the definition and importance of clinical data management, exploring its crucial role in shaping the future of healthcare.”
Creating a Data Management Plan: The Foundation of Successful Clinical Research
Imagine you’re a researcher on a mission to develop a new cancer treatment. You’ve got a talented team, a clear hypothesis, and a robust study design. But, have you considered how you’ll manage the vast amounts of data you’ll collect? A well-crafted data management plan (DMP) is the unsung hero of clinical research, ensuring your data is accurate, secure, and readily available for analysis. Clinical Data Management
Real-Life Example:
In 2019, the National Institutes of Health (NIH) launched the All of Us research program, aiming to collect health data from 1 million participants. To manage this vast dataset, researchers created a comprehensive DMP, outlining data collection, storage, and sharing procedures. This plan enabled them to ensure data quality, privacy, and accessibility, paving the way for groundbreaking discoveries. Clinical Data Management
Key Considerations:
When creating a DMP, consider the following essential elements:
- Data Sources: Identify all data sources, including electronic health records (EHRs), patient-reported outcomes (PROs), and lab results.
- Data Collection Methods: Determine how data will be collected, such as through surveys, wearable devices, or medical imaging.
- Data Standards: Establish consistent data formatting and coding to facilitate analysis and sharing.
- Data Security: Implement measures to protect patient privacy and prevent data breaches.
- Data Sharing: Outline procedures for data access, use, and sharing among researchers and stakeholders.
By investing time and effort into creating a comprehensive DMP, researchers can ensure that their data is a valuable asset, rather than a liability. In the next section, we’ll explore the importance of data quality and integrity in clinical research. Clinical Data Management
Designing Data Collection Tools: The Art of Capturing High-Quality Data
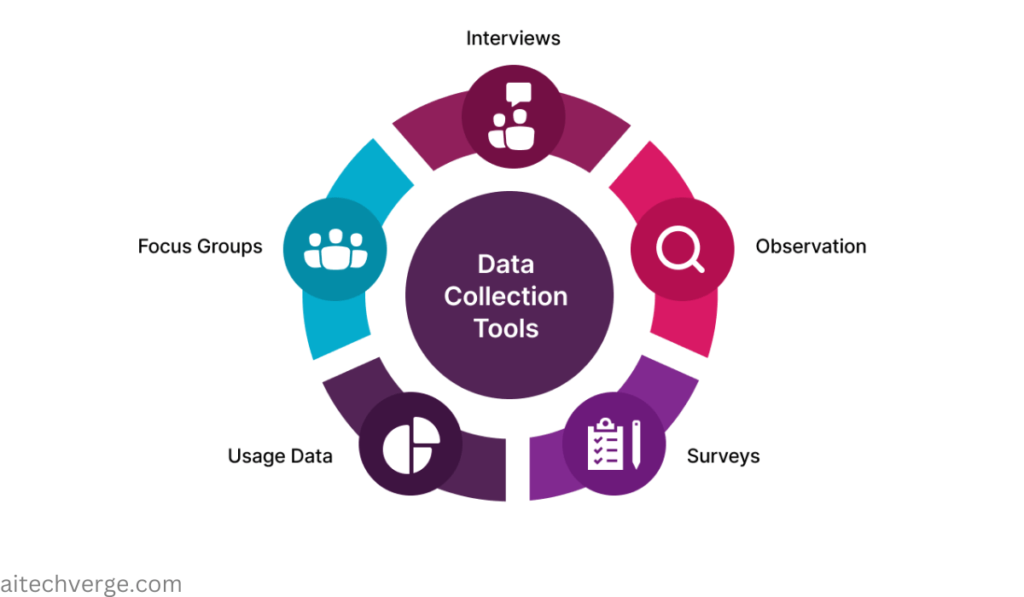
Meet Sarah, a clinical researcher studying the effects of a new diabetes medication. She knows that accurate data collection is crucial to understanding the treatment’s efficacy and safety. But, have you ever wondered how researchers like Sarah design data collection tools that ensure high-quality data? Clinical Data Management
Real-Life Example:
In a recent study on asthma management, researchers created a custom mobile app for patients to track their symptoms and medication use. The app featured a simple, user-friendly interface and reminders to ensure consistent data entry. This thoughtful design led to a remarkable 90% patient adherence rate, providing valuable insights into asthma management. Clinical Data Management
Key Considerations:
When designing data collection tools, keep the following essential elements in mind:
- Clear Objectives: Define what data you need to answer your research questions.
- User-Centered Design: Create tools that are intuitive, easy to use, and accommodate diverse patient populations.
- Standardization: Use standardized formats and coding to facilitate data analysis and sharing.
- Data Validation: Implement checks to detect errors or inconsistencies in real-time.
- Patient Burden: Minimize the data collection burden on patients to ensure high adherence rates.
By carefully crafting data collection tools, researchers like Sarah can ensure that their data is accurate, reliable, and informative. This attention to detail paves the way for meaningful insights and discoveries that can improve human lives. Clinical Data Management
Data Quality Control: The Guardian of Data Integrity
Even with well-designed tools, data quality issues can arise. That’s why researchers must implement robust quality control measures to detect and address errors or inconsistencies.
- Data Cleaning: Regularly review and correct data errors or inconsistencies.
- Data Verification: Validate data against external sources or reference datasets.
- Data Quality Metrics: Establish metrics to monitor data quality and identify areas for improvement.
By combining thoughtful data collection tool design with rigorous quality control measures, researchers can trust their data to tell an accurate story, leading to breakthroughs and improved patient outcomes. Clinical Data Management
Selecting a Data Management System: The Backbone of Secure Data Storage
Meet Dr. Patel, a principal investigator leading a multicenter clinical trial on cardiovascular disease. She knows that storing sensitive patient data requires a robust data management system (DMS) that prioritizes security and compliance. But, with numerous options available, how does she choose the right one? Clinical Data Management
Real-Life Example:
The University of California, San Francisco (UCSF) implemented a cloud-based DMS for their clinical research studies. The system featured advanced security measures, such as encryption and access controls, to protect patient data. This enabled researchers to focus on their work, confident that their data was secure and compliant with regulations.
Key Considerations:
When selecting a DMS, consider the following essential elements:
- Security Features: Ensure the system has robust access controls, encryption, and audit logs.
- Compliance: Verify that the system meets relevant regulations, such as HIPAA or GDPR.
- Scalability: Choose a system that can grow with your research needs.
- User Interface: Opt for an intuitive interface that streamlines data management tasks.
- Integration: Consider a system that integrates with existing tools and platforms.
Data Privacy and Security: The Guardian of Trust
Protecting patient privacy and data security is a top priority in clinical research. Researchers must ensure that their DMS includes robust measures to prevent data breaches and unauthorized access.
- Data Encryption: Ensure data is encrypted both in transit and at rest.
- Access Controls: Implement role-based access controls to limit data access.
- Audit Logs: Regularly monitor system activity to detect potential security incidents.
- Data Anonymization: Use techniques like pseudonymization or anonymization to protect patient identities.
By selecting a robust DMS and prioritizing data privacy and security, researchers like Dr. Patel can focus on their work, confident that their data is secure and compliant with regulations. Clinical Data Management
Data Validation: The Gatekeeper of Data Quality
Meet Emily, a clinical research coordinator, who knows that accurate data is crucial for drawing meaningful conclusions. She meticulously reviews patient records, ensuring that data entry errors don’t compromise the integrity of the study. But, have you ever wondered how Emily validates data to ensure its accuracy? Clinical Data Management
Real-Life Example:
In a recent study on pediatric asthma, researchers used data validation rules to detect inconsistencies in patient-reported symptoms and medication use. For instance, if a patient reported using an inhaler, but the dosage field was left blank, the system would flag the error, prompting Emily to clarify the information. Clinical Data Management
Key Considerations:
When validating data, consider the following essential elements:
- Data Type Checks: Verify that data is in the correct format (e.g., dates, numbers).
- Range Checks: Ensure data falls within expected ranges (e.g., age, blood pressure).
- Consistency Checks: Detect inconsistencies across related data fields.
- Data Normalization: Standardize data formatting to facilitate analysis.
Data Verification: The Double-Check
Even with validation rules in place, errors can still occur. That’s why data verification is crucial.
- Manual Review: Emily reviews data for accuracy, detecting errors that may have slipped through.
- Automated Checks: Use algorithms to detect anomalies and outliers.
Handling Missing or Inconsistent Data: The Art of Data Harmonization
Sometimes, despite best efforts, data may be missing or inconsistent. This is where data harmonization comes in.
- Data Imputation: Use statistical methods to fill in missing values.
- Data Reconciliation: Resolve inconsistencies across data sources.
- Data Transformation: Convert data formats to facilitate analysis.
By validating, verifying, and harmonizing data, Emily ensures that her study’s findings are built on a foundation of high-quality data, ultimately leading to more reliable conclusions and better patient outcomes. Clinical Data Management
Unlocking Insights: Statistical Analysis, Data Visualization, and Reporting
Meet David, a biostatistician, who loves unraveling the stories hidden within data. He’s working on a clinical trial analyzing the effectiveness of a new diabetes treatment. David knows that statistical analysis, data visualization, and reporting are crucial steps in transforming data into actionable insights. Clinical Data Management
Real-Life Example:
In a recent study on cardiovascular disease, researchers used statistical analysis to identify high-risk patient groups. They created interactive visualizations to explore how different factors, such as age and blood pressure, impacted disease progression. These findings were then reported clearly and concisely, informing healthcare professionals and patients about personalized treatment strategies. Clinical Data Management
Key Considerations:
When analyzing and reporting data, consider the following essential elements:
- Statistical Analysis: Select appropriate techniques (e.g., regression, survival analysis) to answer research questions.
- Data Visualization: Use plots and charts to illustrate findings, facilitating understanding and exploration.
- Reporting: Communicate results, including data visualizations, in a transparent and reproducible manner.
Data Visualization: The Art of Communication
Effective data visualization is critical for communicating complex findings to various audiences.
- Exploratory Data Analysis: Use visualization to understand data distribution, outliers, and relationships.
- Results Visualization: Present findings clearly and concisely, using appropriate plots and charts.
- Interactive Visualization: Enable users to explore data in real-time, fostering deeper understanding and insights.
Reporting: The Bridge to Actionable Insights
Clear and concise reporting is essential for translating findings into actionable recommendations.
- Results Summary: Provide an overview of key findings, including statistical significance and effect sizes.
- Methodological Transparency: Describe data collection, analysis, and visualization methods.
- Conclusion and Recommendations: Offer actionable insights and guidance for future research or clinical practice.
By leveraging statistical analysis, data visualization, and reporting, David uncovers meaningful patterns and insights, ultimately improving patient outcomes and advancing healthcare knowledge. Clinical Data Management
Navigating Regulatory Waters: Adhering to Requirements and Ensuring Data Transparency
Meet Rachel, a clinical research manager, who knows that navigating regulatory requirements is crucial for ensuring the integrity and validity of clinical trials. She’s working on a study involving a new medical device and must ensure that her team complies with relevant regulations, such as FDA guidelines. Rachel understands that data transparency is essential for building trust with patients, researchers, and regulatory agencies. Clinical Data Management
Real-Life Example:
In a recent study on Alzheimer’s disease, researchers ensured data transparency by implementing a data-sharing platform. This allowed them to share de-identified data with other researchers, fostering collaboration and accelerating discovery. Additionally, they adhered to regulatory requirements by obtaining informed consent from patients and maintaining accurate records.
Key Considerations:
When adhering to regulatory requirements and ensuring data transparency, consider the following essential elements:
- Regulatory Compliance: Familiarize yourself with relevant regulations, such as FDA guidelines, HIPAA, and GDPR.
- Data Privacy: Implement measures to protect patient privacy and maintain data confidentiality.
- Data Sharing: Establish policies for data sharing, ensuring transparency and collaboration.
- Transparency Reporting: Regularly report on study progress, results, and any issues that arise.
Data Transparency: The Cornerstone of Trust
Data transparency is critical for building trust with patients, researchers, and regulatory agencies.
- Data Access: Provide secure access to data for authorized personnel and researchers.
- Data Documentation: Maintain accurate and detailed documentation of data collection, management, and analysis.
- Data Auditing: Regularly audit data to ensure accuracy, completeness, and integrity.
Regulatory Requirements: The Guardian of Integrity
Adhering to regulatory requirements ensures the integrity and validity of clinical trials.
- Informed Consent: Obtain informed consent from patients, ensuring they understand the risks and benefits.
- Data Quality: Implement measures to ensure data quality, accuracy, and reliability.
- Regulatory Submissions: Prepare and submit regulatory documents, such as IND applications and study protocols.
By navigating regulatory requirements and ensuring data transparency, Rachel ensures that her study is conducted with the highest ethical standards, building trust and advancing medical knowledge. Clinical Data Management
Unlocking the Power of Collaboration: Sharing Data and Insights
Meet Dr. Maria, a principal investigator, who believes that sharing data and collaborating with stakeholders is essential for advancing medical research. She’s working on a study investigating the genetic factors of rare diseases and recognizes that sharing data with other researchers, clinicians, and patients is crucial for driving progress.
Real-Life Example:
In a recent study on cystic fibrosis, researchers shared their data and collaborated with clinicians, patients, and industry partners. This led to the development of a new treatment, approved by the FDA, which has improved the lives of thousands of patients. Clinical Data Management
Key Considerations:
When sharing data and collaborating on analysis and interpretation, consider the following essential elements:
- Data Sharing: Establish clear policies and procedures for sharing data with stakeholders.
- Collaboration: Foster open communication and collaboration among researchers, clinicians, patients, and industry partners.
- Data Standardization: Ensure data is standardized and interoperable to facilitate sharing and analysis.
- Intellectual Property: Address intellectual property considerations and ensure that data sharing and collaboration align with institutional policies.
Collaborative Analysis and Interpretation: The Key to Unlocking Insights
Collaborative analysis and interpretation of data lead to more comprehensive and accurate insights.
- Diverse Perspectives: Bring together researchers, clinicians, patients, and industry partners to provide diverse perspectives and expertise.
- Data Integration: Integrate data from multiple sources to gain a more complete understanding of the research question.
- Insight Generation: Collaborate on analysis and interpretation to generate new insights and discoveries.
Stakeholder Engagement: The Foundation of Trust
Engaging stakeholders in data sharing and collaboration builds trust and ensures that research is patient-centric.
- Patient Engagement: Involve patients in the research process, ensuring their voices are heard and their needs are addressed.
- Clinician Engagement: Collaborate with clinicians to ensure research is relevant and applicable to clinical practice.
- Industry Engagement: Partner with industry partners to leverage their expertise and resources.
By sharing data and collaborating on analysis and interpretation, Dr. Maria and her team can unlock new insights, drive progress, and improve patient outcomes. Clinical Data Management
Preserving the Past, Informing the Future: Ensuring Long-Term Data Preservation
Meet Dr. Lee, a researcher who understands the importance of preserving data for future generations. She’s working on a study about climate change and knows that the data she collects today will be invaluable for researchers in the years to come. Clinical Data Management
Real-Life Example:
The National Center for Atmospheric Research (NCAR) has a data archive that stores climate data from research studies spanning over 40 years. This archive has been instrumental in advancing our understanding of climate change and has informed policy decisions worldwide.
Key Considerations:
When ensuring long-term data preservation and adhering to retention policies, consider the following essential elements:
- Data Storage: Select appropriate data storage solutions that ensure data integrity and accessibility.
- Data Format: Use standardized data formats to facilitate data sharing and analysis.
- Metadata: Create detailed metadata to provide context and understanding of the data.
- Data Backup: Regularly backup data to prevent loss due to technical issues or natural disasters.
- Retention Policies: Adhere to institutional and legal retention policies to ensure data is kept for the required amount of time.
Data Preservation: The Guardian of Scientific Integrity
Ensuring long-term data preservation is crucial for maintaining scientific integrity and advancing research.
- Data Sharing: Share data with other researchers to facilitate collaboration and verification.
- Data Reuse: Allow data to be reused in new research studies, promoting efficiency and innovation.
- Data Legacy: Ensure data is preserved for future generations, providing a foundation for continued discovery.
Retention Policies: The Framework for Responsible Data Management
Adhering to retention policies ensures that data is managed responsibly and in compliance with legal and institutional requirements.
- Data Classification: Classify data based on sensitivity and importance to determine appropriate retention periods.
- Data Destruction: Ensure data is properly destroyed when it reaches the end of its retention period.
- Compliance: Adhere to legal and institutional retention policies to avoid data-related risks.
By prioritizing long-term data preservation and adhering to retention policies, Dr. Lee and her team can ensure that their research contributes to a lasting legacy of scientific progress. Clinical Data Management
The Future of Clinical Research: Harnessing the Power of Clinical Data Management
As we’ve explored throughout this article, Clinical Data Management (CDM) is the backbone of clinical research. It ensures that data is accurate, reliable, and accessible, ultimately driving better patient outcomes. Let’s recap the importance of CDM and gaze into the future of clinical research. Clinical Data Management
Recap: Why CDM Matters
- Ensures data quality and integrity
- Enables data sharing and collaboration
- Supports regulatory compliance
- Facilitates data analysis and insights
- Drives better patient outcomes
Future Directions:
- Artificial Intelligence (AI) and Machine Learning (ML) Integration: Leveraging AI and ML to automate data cleaning, data transformation, and data analysis.
- Cloud-Based CDM: Migrating CDM systems to the cloud for enhanced scalability, security, and collaboration.
- Patient-Centric CDM: Prioritizing patient privacy, consent, and engagement in CDM processes.
- Real-World Data (RWD) Integration: Incorporating RWD into CDM systems to expand research capabilities.
- Global CDM Standards: Establishing universal CDM standards for seamless collaboration and data sharing.
Real-Life Example:
The National Institutes of Health (NIH) has launched the “All of Us” research program, aiming to collect health data from 1 million participants. This initiative relies on robust CDM practices to ensure data quality, security, and accessibility. Clinical Data Management
Human Touch:
CDM is not just about data; it’s about people. It’s about ensuring that patients’ data is handled with care and respect. It’s about empowering researchers to make groundbreaking discoveries. And it’s about driving better patient outcomes. Clinical Data Management
As we look to the future of clinical research, let’s prioritize CDM as the foundation upon which we build. By doing so, we’ll unlock new possibilities for scientific discovery and improved patient care. Clinical Data Management

